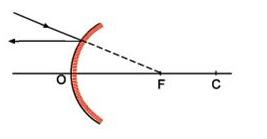
Thermodynamics
Thermodynamics States About Energy Conversion Thermodynamics is the branch of science that embodies the principles of energy transformation in macroscopic systems. The general restrictions which experience has shown to apply to all such transformations are known as the laws of thermodynamics. These laws are primitive; they cannot be derived from anything more basic. The first law of thermodynamics states that energy is conserved; that, although it can be altered in form and transferred from one place to another, the total quantity remains constant. Thus, the first law of thermodynamics depends on the concept of energy; but, conversely, energy is an essential thermodynamic function because it allows the first law to be formulated. This coupling is characteristic of the primitive concepts of thermodynamics. The words system and surroundings are similarly coupled. A system is taken to be any object, any quantity of matter, any region, and so on, selected for study and set apart (men...